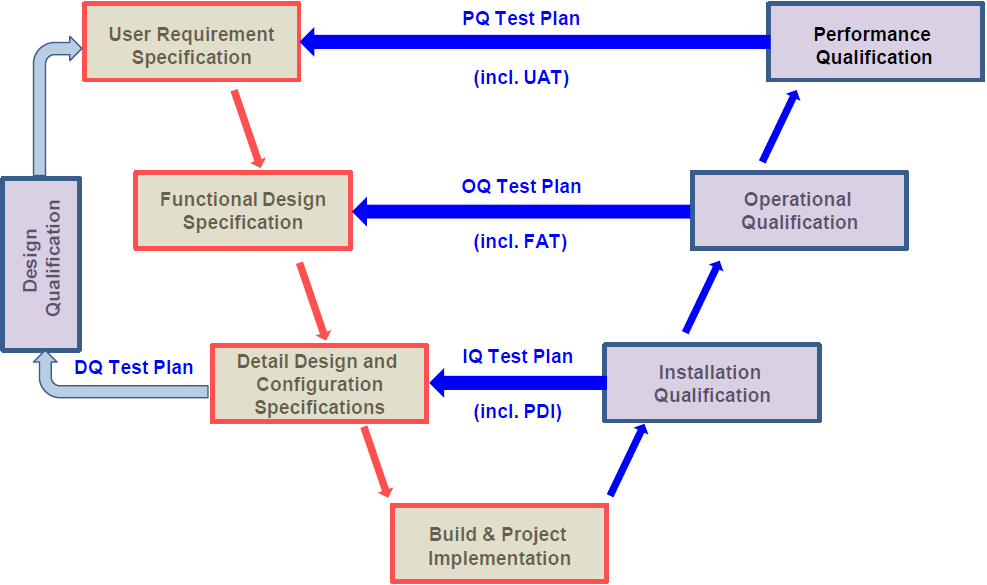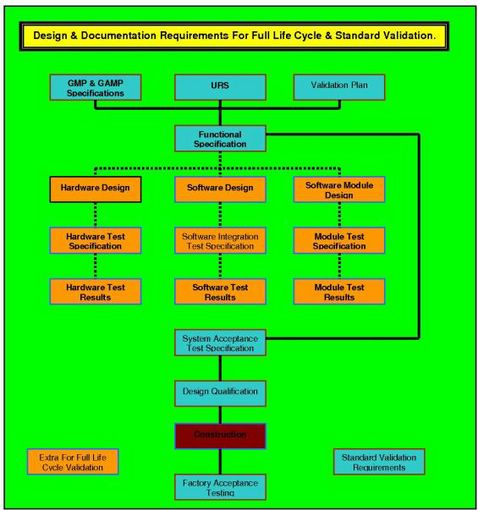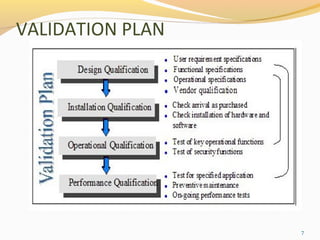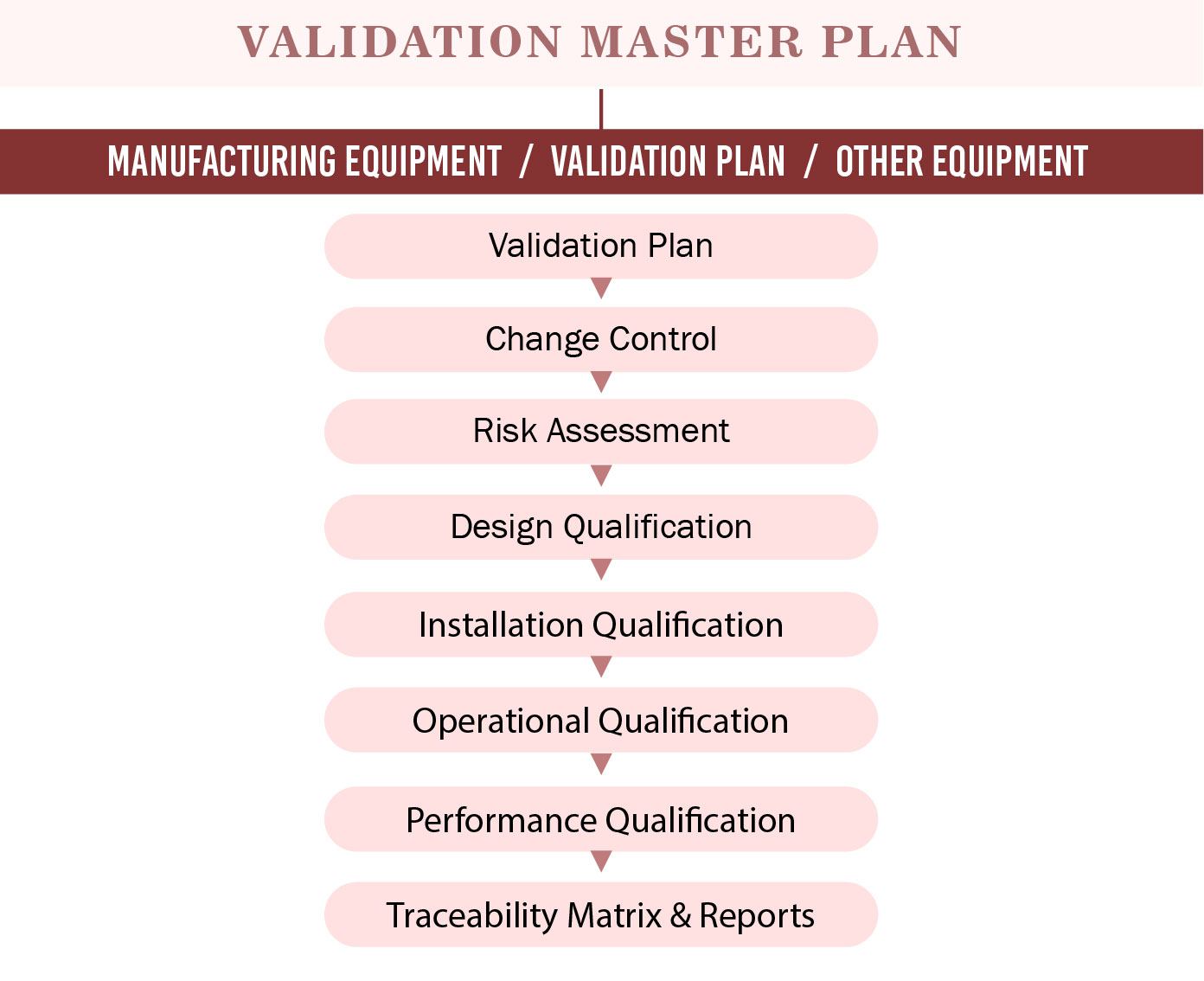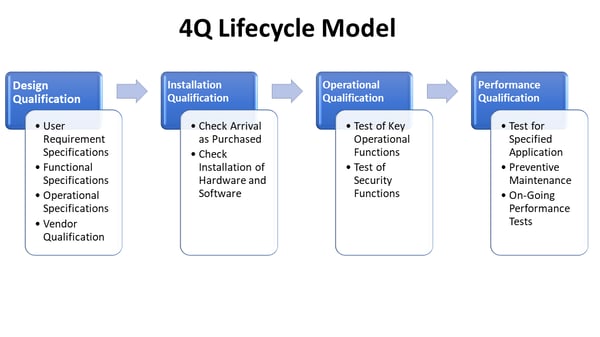
Recommended Best Practices for Lyophilization Validation 2021 Part II: Process Qualification and Continued Process Verification | SpringerLink

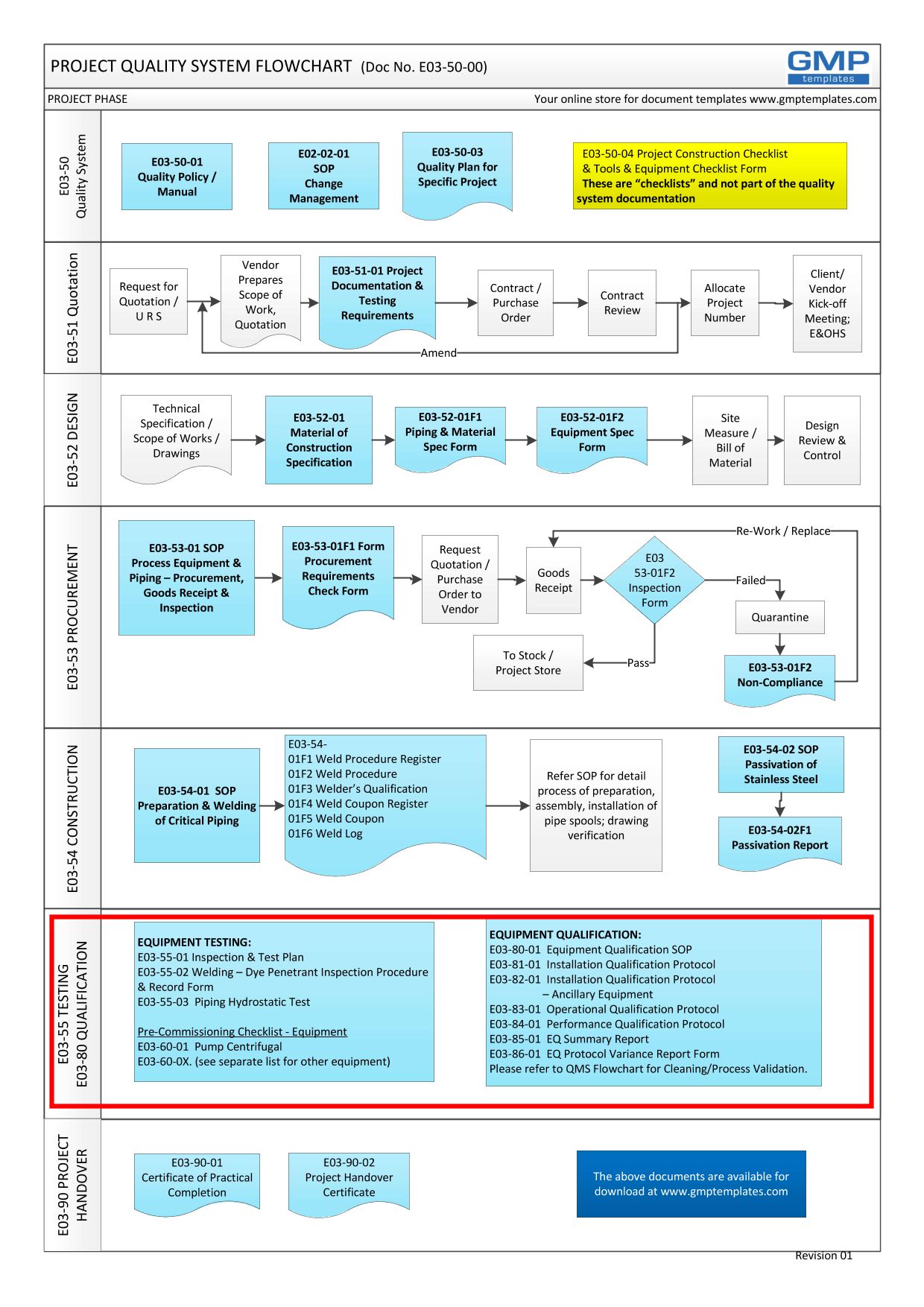
Pharma By Air: Equipment Qualification and Route Risk Assessment | Pharmaceutical Outsourcing - The Journal of Pharmaceutical & Biopharmaceutical Contract Services

Guidance on Qualification of existing facilities, systems, equipment and utilities - PDF Free Download