Towards new methods for the determination of dose limiting toxicities and the assessment of the recommended dose for further studies of molecularly targeted agents – Dose-Limiting Toxicity and Toxicity Assessment Recommendation Group

Designing Dose-Finding Phase I Clinical Trials: Top 10 Questions That Should Be Discussed With Your Statistician | JCO Precision Oncology

Innovative design for a phase 1 trial with intra-patient dose escalation: The Crotoxin study. - Abstract - Europe PMC

Heterogeneity in the definition of dose-limiting toxicity in phase I cancer clinical trials of molecularly targeted agents: a review of the literature. | Semantic Scholar

Innovative design for a phase 1 trial with intra-patient dose escalation: The Crotoxin study - ScienceDirect

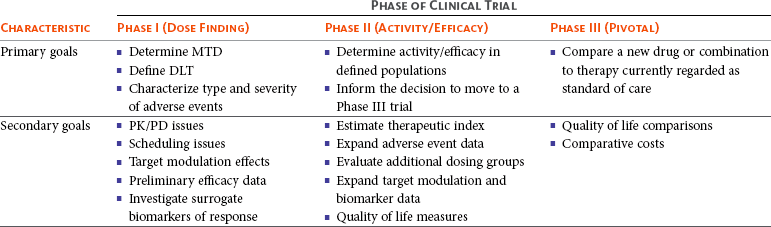
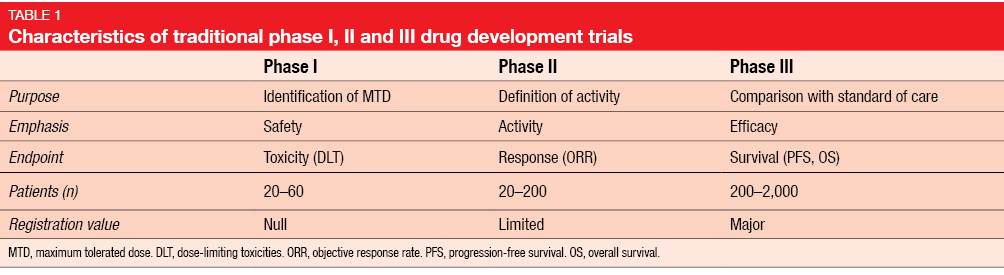
Safety and Efficacy of a Five-Fraction Stereotactic Body Radiotherapy Schedule for Centrally Located Non–Small-Cell Lung Cancer: NRG Oncology/RTOG 0813 Trial | Journal of Clinical Oncology

Towards new methods for the determination of dose limiting toxicities and the assessment of the recommended dose for further studies of molecularly targeted agents – Dose-Limiting Toxicity and Toxicity Assessment Recommendation Group

Immune checkpoint inhibitor-based combinations: is dose escalation mandatory for phase I trials? - Annals of Oncology
Application Type sNDA Application Number(s) 201023/S-20 Priority or Standard Priority Submit Date(s) November 21, 2016 Received
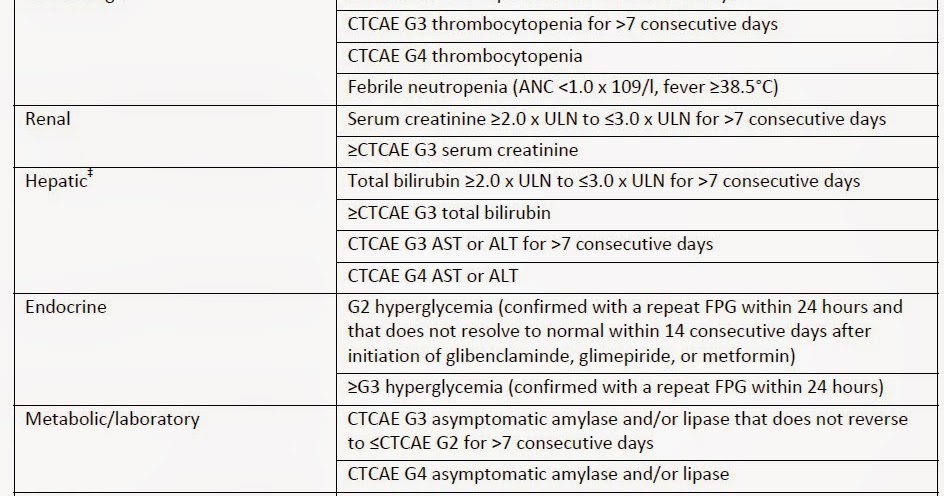
On Biostatistics and Clinical Trials: Dose Limiting Toxicity (DLT) and Common Toxicity Criteria (CTC) / Common Terminology Criteria for Adverse Events (CTCAE)

Illustration of the chronic dose-limiting toxicity (DLT) concept. (*)... | Download Scientific Diagram
Dose Limiting Toxicities (DLT) DOSE LIMITING TOXICITIES (DLT) Example: Dose escalation will proceed within each cohort according