Reviewing the role of healthy volunteer studies in drug development | Journal of Translational Medicine | Full Text

Moving Beyond 3+3: The Future of Clinical Trial Design | American Society of Clinical Oncology Educational Book

Prediction of Drug Approval After Phase I Clinical Trials in Oncology: RESOLVED2 | JCO Clinical Cancer Informatics

Designs of drug-combination phase I trials in oncology: a systematic review of the literature - Annals of Oncology

Illustration of the chronic dose-limiting toxicity (DLT) concept. (*)... | Download Scientific Diagram

On Biostatistics and Clinical Trials: Dose Limiting Toxicity (DLT) and Common Toxicity Criteria (CTC) / Common Terminology Criteria for Adverse Events (CTCAE)
Guidance for Industry Acute Myeloid Leukemia: Developing Drugs and Biological Products for Treatment
Application Type sNDA Application Number(s) 201023/S-20 Priority or Standard Priority Submit Date(s) November 21, 2016 Received
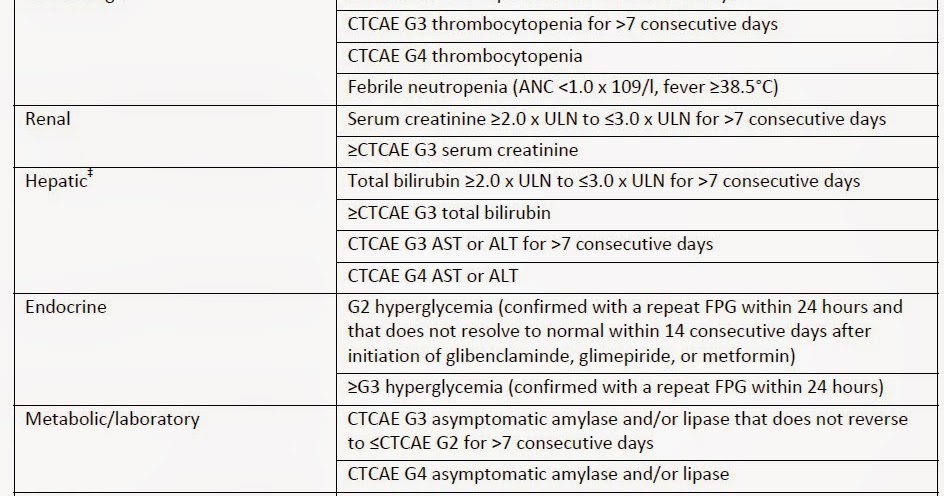
On Biostatistics and Clinical Trials: Dose Limiting Toxicity (DLT) and Common Toxicity Criteria (CTC) / Common Terminology Criteria for Adverse Events (CTCAE)

ELIZABETH GARRETT-MAYER (SOME SLIDES BY PAT LORUSSO OF KARMANOS CANCER INSTITUTE WAYNE STATE UNIVERSITY) Phase I Trials of Chemotherapy and Targeted Agents. - ppt download

On Biostatistics and Clinical Trials: Dose Limiting Toxicity (DLT) and Common Toxicity Criteria (CTC) / Common Terminology Criteria for Adverse Events (CTCAE)

Towards new methods for the determination of dose limiting toxicities and the assessment of the recommended dose for further studies of molecularly targeted agents – Dose-Limiting Toxicity and Toxicity Assessment Recommendation Group