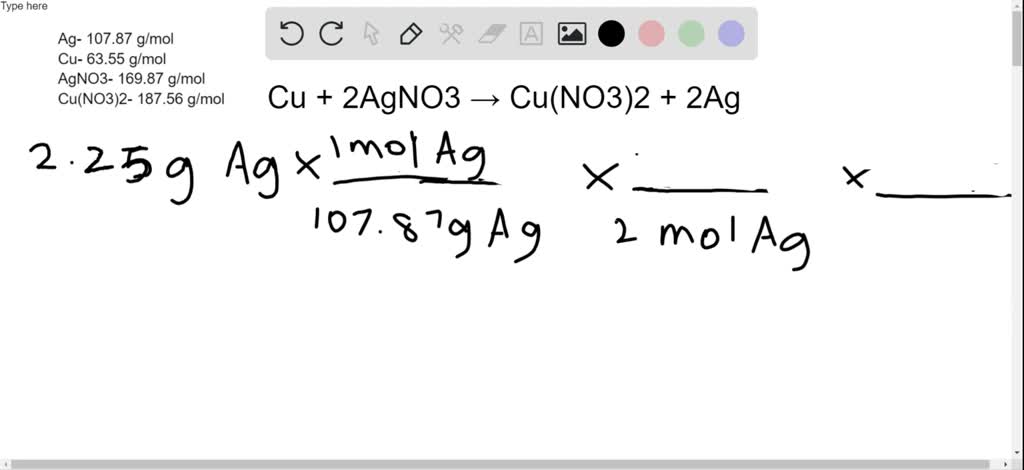
Write balanced chemical equations for these equations : Silver is precipitated out when a copper strip is dipped in silver nitrate solution. The solution turns blue due to the formation of copper (

Conservation of Mass & Word Equations. Demonstration Mass of apparatus and liquids in test tubes Before chemical reaction:______ After chemical. - ppt download

Write the balanced chemical equations for the following reaction:Calcium hydroxide + Carbon dioxide → Calcium Carbonate + Water.

Word Equations & Predicting Products Mrs. Cook. Write a balanced chemical equations for this reaction. A solution of barium hydroxide reacts with a sulfuric. - ppt download

In the refining of silver, the recovery of silver from silver nitrate solution involved displacement by copper metal. Write down the reaction involved.